FOR RESEARCHERS
Major challenges in the field include ill-defined technical standards for disease data acquisition and sharing, limits in databases to correlate phenotypes with genomic variations and a scarcity of in vitro and in vivo models to study the underlying mechanism.
These challenges are addressed by RareBoost. Below, we provide a preliminary outline of our scientific approach, which will be revised with the ERA Chair holder.
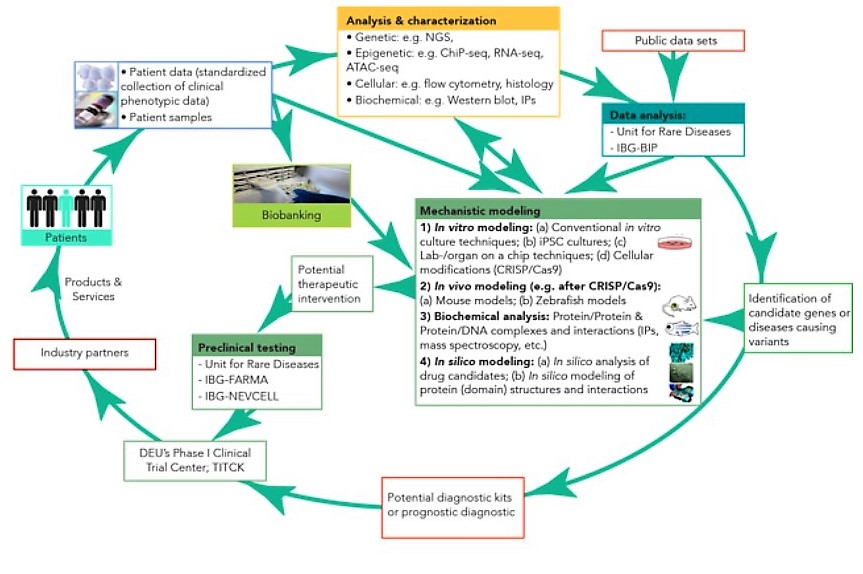
(1) Deep phenotyping to determine genetic factors:
‘Deep phenotyping’ is defined as the precise and comprehensive analysis of the phenotypic and genomic alterations observed in individual patients. This precision medicine approach is particularly relevant for rare diseases as the diagnosis and therapy is personalized for each patient’s genetic alterations.
The phenotypic data set comprises the standardized collection of the clinical parameters of the disease and its pathology, including a comprehensive analysis of cellular and biochemical changes.
The genomic data set will include, on the one hand, the screen for DNA variants with a genome-wide scope using different next-generation sequencing (NGS)-based methodologies. On the other hand, we will investigate the potential epigenetic regulation of rare diseases, including histone variants, long non-coding RNAs and DNA methylation.
Using these patient-derived data, comprehensive in silico approaches will identify novel candidates for disease-causing genetic or epigenetic aberrations and pathways.
(2) Advanced in vitro/vivo technologies to uncover the underlying mechanisms:
Once candidate genes and pathways are identified, experimental approaches will be utilized to determine their underlying biological mechanisms.
This will be done, on the one hand, by advanced 2D/3D cell culture experiments and lab-/organ-on-a-chip (LOC) culture techniques. As LOC experiments use primary cells and tissues from individual disease patients, they provide a more physiological environment than traditional culture techniques, and allow, therefore, an improved modelling of the pathology. Furthermore, in some cases, cells will be tested in the cell culture experiments that have been genetically altered via CRISPR/Cas9-technology.
On the other hand, transgenic animal models will be utilized, as they are invaluable for the mechanistic understanding of rare diseases and for the development of novel therapies. However, for most rare diseases, suitable animal models are currently not available. We envision that zebrafish and mouse models will be particularly useful, and the required facilities are already operational at IBG. The zebrafish models are anticipated to be primarily used for highly conserved, essential cell biology genes. In contrast, genetic mouse models are anticipated to be particularly useful to model complex, systemic phenotypes.
These in vitro, ex vivo and in vivo experimental models will allow the RareBoost researchers to uncover the underlying mechanisms of the candidate genes/pathways.
(3) Translation of research findings to devising novel diagnostic and therapeutic approaches:
The gained mechanistical understanding for particular rare diseases will aid the development of new or improved diagnostic approaches or prognostic and therapeutic strategies.
First, the identification of disease-causing mutations or modifications is expected to allow us to develop new diagnostic panels for the early detection of rare diseases.
Second, knowledge of the underlying biological mechanisms of rare diseases will point to novel therapeutic targets. This will be aided by IBG’s computational structural biology research groups by in silico tools to search for drug candidates. The efficacy and toxicity of such candidates can be tested by LOC culture techniques or in developed animal models.
Third, the infrastructure and expertise required to verify diagnostic or therapeutic candidates in preclinical studies and to prepare them for clinical studies is already established at IBG’s technology transfer platforms.
Despite the collective expertise of IBG’s researchers, there are far too many rare diseases for us to cover them all. Therefore, IBG invites basic, clinical and industry researchers, working on all aspects of rare diseases, to collaborate with us.